





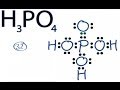








H3PO4. Within the Brønsted–Lowry acid-base theory (protonic), a conjugate acid is the acid member, HX, of a pair of two compounds that transform into each other by gain or loss of a proton (hydrogen ion). The conjugate base of H2PO4... chemistry. The conjugate base of H 2 P O 4 − is: A. H P O 4 2 − B. P 2 O 5 C. H 3 P O 4 D. P O 4 3 − Medium. Answer. The concept of conjugate Acid-Base pair was given by Lowry-Bronsted Theory. According to the theory: Acids are proton (H +) donors. The conjugate system involves proton transfer. The conjugate acid of H2PO4- is H3PO4 or phosphoric acid with a added proton. If it is conjugate acid, add a proton then remove a negative charge. If it is conjugate base, remove a proton then add a negative charge. The conjugate base of H2PO4... chemistry. The conjugate base of H 2 P O 4 − is: A. H P O 4 2 − B. P 2 O 5 C. H 3 P O 4 D. P O 4 3 − MEDIUM. Answer. The concept of conjugate Acid-Base pair was given by Lowry-Bronsted Theory. According to the theory: Acids are proton (H +) donors. The conjugate base of an acid, any acid, is defined as the acid "LESS" a proton, H^+. The conjugate acid of a base, any base, is defined as the base "PLUS" a proton. Phosphoric acid, H_3PO_4, is the parent acid. If it loses a proton, H^+, we conserve both mass and charge, and H_2PO_4^- results. And what is the conjugate base of this beasty? In H2O the conjugate base is H2PO4-, being conjugated to the acid H3PO4. As well: H3PO4 is conjugated acid to the base H2PO4-. The H2PO4- ion has both a conjugate acid and a conjugate base. It is true that A) H3PO4 is the conjugate acid. B) HPO42- is the conjugate base. C) H2PO3- is the conjugate base. D) more than one correct response E) no correct response Similarly, what is the conjugate base of the Bronsted Lowry acid hpo4 2 -? Hence an acid donates a proton to form it's corresponding conjugate base while a base accepts a proton to form it's corresponding conjugate acid. From Bronsted Lowry theory , we can conclude that the conjugate base of HPO4^-2 is PO4^-3. Which of the following is the conjugate base of HPO42-? a. H2O b. H3PO4 c. H2PO4- d. PO43- e. P2O5? Conjugate acid and base 1 answer below » In the reaction, H2PO4- + HAsO42- HPO42- + H2AsO4-, which species are a conjugate acid-base pair? Feb 21 2012 11:05 AM
[index] [3471] [7165] [9633] [690] [7127] [1297] [4161] [59] [3722] [1944]
We've all heard the terms acid and base. What do these mean? Don't just tell me about pH, silly. What structural detail makes a molecule an acid or a base? Y... In the Brønsted-Lowry definition of acids and bases, a conjugate acid-base pair consists of two substances that differ only by the presence of a proton (H⁺).... Determine acid/base ratio of a buffer About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ... A step-by-step explanation of how to draw the HCOOH Lewis Structure Methanoic Acid (Formic Acid).For the HCOOH Lewis structure, calculate the total number of... A step-by-step explanation of how to draw the H3PO4 Lewis Dot Structure (Phosphoric acid).For the H3PO4 structure use the periodic table to find the total nu... Learn everything about Conjugate Acids and Bases. We explain this with the real world example of vinegar.At Fuse School, teachers and animators come together... This chemistry video tutorial explains how to calculate the pH of a buffer solution using the henderson hasselbalch equation. It explains the concept, compo...
Copyright © 2024 top100.toprealmoneygames.xyz